WHY DO WE WANT EVEN HEATING AND WHAT CAN WE DO ABOUT IT?
The main job of the stovetop cookware is to smooth out the uneven heat coming from the burner underneath so that the cooking surface of the cookware is the same temperature. If you have too much of an imbalance in temperatures, you wind up with hot or cold spots that can undermine your dish and your health by leaving some food overcooked and some food undercooked. You may even scorch oil and produce carcinogenic smoke, if some hotspots grow hotter than the oil’s smoke point. If you’ve ever cooked fish where part of a fillet got overcooked while the rest was undercooked; if you’ve ever made rice and had some undercooked while the rest burned; if you’ve ever burned part of a strip of bacon while waiting for the ends of the strips to be done, then you’ve already experienced the joy of uneven heating.
A second job of cookware is to hold that heat so as to maximize Maillard reactions. These reactions take place at higher temperatures. Thus if you have a flimsy pan and tossing in a steak crashes the temperature, that will mean more steaming/boiling of your food and lower Maillard reactions, resulting in less-tasty food.
The larger the diameter of your cookware bottom relative to the diameter of the flame or heating element or induction coil, the bigger the uneven heating problem can be, since heat has to travel a longer distance to reach the sides. (This is less true of gas, since hot gases can roll up the sides in all directions and naturally smooths out heat somewhat, but as we shall see in another article, the problem can persist. Also, gas and induction rings can produce a problem where the pan’s center is colder than the hot circle directly above the gas/induction ring.)
There are various ways you can attempt to fix the problem of uneven heating:
1. Buy a bigger burner. If your current burners aren’t cutting it, you can go shopping for a new cooktop/range, but that can be expensive. (I have written an in-depth discussion of various cooktop/range technologies.) You can also buy portable gas, electric, and induction burners, but most cheap portable burners will have smaller effective areas than full-size cooktops/ranges.
2. Buy better cookware. This can get expensive, but if you buy durable cookware, you effectively spread the cost out over many decades. I will be posting my measurements of various cookwares’ even heating ability on induction/electric. Gas results are also available.
3. Continually move your food. Continual stirring ensures that each ingredient spends time in the hot spots. This method works for some foods, but you run into problems if you have to tend to multiple tasks in the kitchen at the same time or get distracted, say, by a pet or kid or cookbook. Furthermore, you can miss spots like the hard-to-reach area at the edges, like chasing peas around the perimeter with your spatula. Some food can’t really be stirred, such as eggs and fish. And some food is sticky and does not like to be prodded continually, like potstickers. In fact, fish and meats like to stick-and-release and make fond (tasty stuck on brown bits packed with Maillard reactions) on surfaces like stainless steel. You disturb that process if you keep stirring.
4. Preheat your pans slowly. This solution is imperfect, because a) cookware radiates heat, so there comes a point where if you heat something too slowly, you might as well not heat it at all because it will lose heat to the surrounding air as fast as you pump in heat from the burner below; b) you waste a lot of time and possibly energy; and c) if you toss cold food onto that pan you babied into evenness, then pan temperature will drop, so you may need to crank up the heat again anyway. For really bad thermal conductors like cast iron, no amount of coddling on the stovetop will make them even heating, but you could preheat them in an oven. However that eats more time/energy/money as oven heating is far less efficient than stovetop heating.
5. Cook everything in the oven or with boiling water or with steam. Ovens heat evenly (heat comes from all sides into the dish). Hot/boiling water or water vapor also heats evenly, because the water or water vapor heats from all sides–eventually, anyway. But not everything can be cooked using these methods, which limits the kinds of foods you can prepare (and how you can prepare them). Furthermore, electric ovens are energy-inefficient ways to cook food, though gas ovens aren’t so bad.
6. Buy a large slab of aluminum or copper to cover up your stovetop burner and thereby make a relatively even heating surface for your cookware. This works in theory, but in practice there are a lot of drawbacks, so I don’t recommend it. The short story is that it takes much longer to heat up stuff, and the copper will develop a matte brown to black crust that is hard to remove, though if you drop boiling water on it while it’s hot, the crust may fracture and reveal the copper underneath. Aluminum won’t develop such a nasty crust, but aluminum melts if you forget about a pot and overheat the aluminum, resulting in melted aluminum dripping down beneath the grate.1
WHY YOU SHOULD BE SKEPTICAL ABOUT CLAIMS THAT ALL COOKWARE IS EVEN HEATING
If you look at online reviews, virtually everybody claims that the cookware they use is even heating. However, they don’t specify what kind of heating levels they use. Low heat on gas is much more forgiving than high heat on induction, for instance. E.g., cast iron heated on very low heat on an electric coil burner as large in diameter as the cast iron may be as even-heating as All-Clad stainless heated on high on a severely undersized single-coil induction burner. But is that fair? Of course not. The deck is stacked in favor of the cast iron in that case.
Even supposedly professional review sites don’t seem to understand thermodynamics. For instance, one site that shall go unnamed reviewed cast iron and used recipes that called for putting the pan into the oven. Based on those tests, they claimed that the cast iron was even heating. But anything thrown into an oven will be even heating, because the oven is subjecting the cookware to heat from all sides! Similarly, water boils evenly in any cookware, because the water itself will move heat around and smooth out any temperature differences at the bottom of the pan.
CONCLUSION
So by now we know that even heating matters because you don’t want a hotspot that will smoke your oil while the rest of the pan isn’t hot enough; or undercook or overcook portions of your food. We also know that heat retention matters for large steaks, fillets of fish, etc. because they will crash the pan temperature down below the range required to produce tasty chemical reactions (Maillard reactions).
How are we to evaluate cookware thermally, then?
What you really want to do when testing cookware for even-heating ability is to stress test it under realistic scenarios. You want realistic tests such as my induction tests and gas tests which subject cookware to medium-low and medium-high heat (about the equivalent of 5900 and 12000 Btu/h). These are not unusual situations for home cooks–most of us have seared steaks or sautéed veggies at medium to high heat before, and we know that you can’t do a proper job of it at low temperatures, so we can’t coddle our cookware using low heat. And most of us don’t cook with big, restaurant-grade gas ranges. Many of us rent or own homes that have undersized burners that don’t heat as evenly.
As for thermal retention, that’s more difficult to test, so I just literally cook large amounts of meat or fish in pans in my product reviews and give a qualitative assessment of how they perform.
I’ll end this entry with some an illustration of how uneven heating looks in a real-life scenario. Below, you can see a 2-minute time lapse of how poorly an enameled oval cast iron dutch oven heats up on medium-low heat (790W induction). Even after two full minutes, the central “O” still has not closed, unlike most other cookware I’ve tested. In comparison, you can see what a pan with 2 mm of copper disc base looks like after just one minute of medium-low heat (790W induction). Cooking food means you need continuous energy being pumped into the cookware, so we can already tell that that cast iron won’t cook as evenly as the copper-based pan.
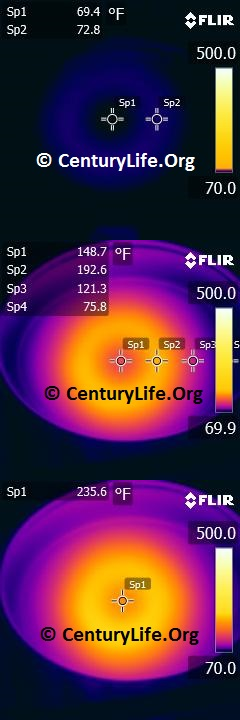
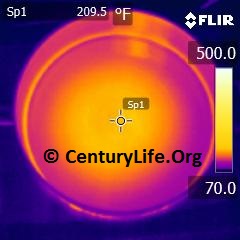
FOOTNOTES



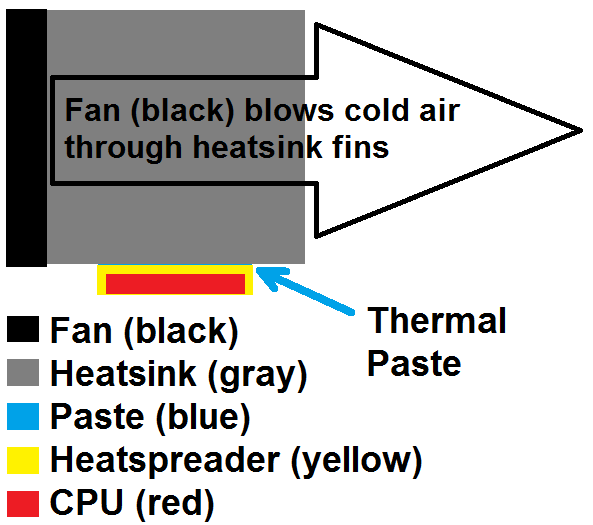 The problem of air gaps between flat metal plates is not unique. In the computer industry, high-performance computer processing units (CPUs) can burn out within minutes if uncooled. Such CPUs are mounted to heatspreaders, which make contact with aluminum or copper heat sinks with fans blowing across the heatsink in order to more quickly dissipate heat. To fill in the air gaps, computer builders use thermal paste ~50 times more heat conductive than air. (Copper is about 13,000 times and aluminum is about 5,300 times more heat conductive than air.) (A typical thermal compound might have thermal conductivity of 1.5 W/(mK), compared to 0.03 W/(mK) for air, 160 W/mK for 3003-H14 aluminum alloy, and about 385 W/mK for copper. Id. Also see
The problem of air gaps between flat metal plates is not unique. In the computer industry, high-performance computer processing units (CPUs) can burn out within minutes if uncooled. Such CPUs are mounted to heatspreaders, which make contact with aluminum or copper heat sinks with fans blowing across the heatsink in order to more quickly dissipate heat. To fill in the air gaps, computer builders use thermal paste ~50 times more heat conductive than air. (Copper is about 13,000 times and aluminum is about 5,300 times more heat conductive than air.) (A typical thermal compound might have thermal conductivity of 1.5 W/(mK), compared to 0.03 W/(mK) for air, 160 W/mK for 3003-H14 aluminum alloy, and about 385 W/mK for copper. Id. Also see